Intro
The importance of accurate labeling in medical settings cannot be overstated. One crucial aspect of this is the labeling of blood bags, which ensures that the right blood products are administered to the right patients at the right time. A blood bag label template is a critical tool in this process, providing a standardized format for recording essential information. In this article, we will explore the 7 essential elements that must be included in a blood bag label template.
Understanding the Importance of Blood Bag Labeling
Blood bag labeling is a critical step in the blood transfusion process. It ensures that the blood product is correctly identified, and its contents are clearly communicated to healthcare professionals. Accurate labeling helps prevent errors, such as transfusing the wrong blood type or administering expired products. A well-designed blood bag label template is essential in reducing the risk of adverse events and ensuring patient safety.
The 7 Essential Elements of a Blood Bag Label Template
A blood bag label template must include the following essential elements to ensure accurate identification and communication of blood products:
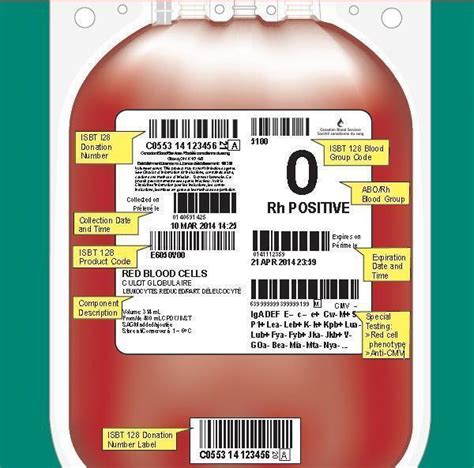
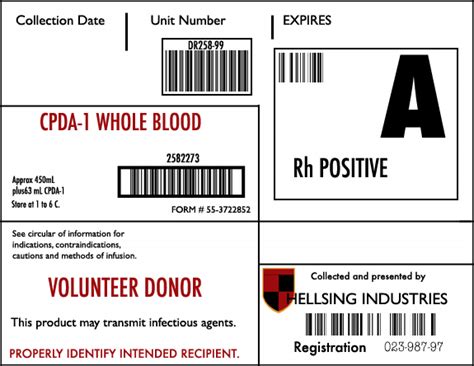
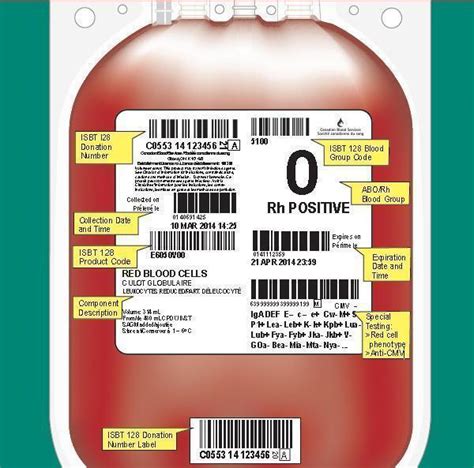
1. Product Identification

The product identification section must include the name and description of the blood product, such as "Whole Blood" or "Red Blood Cells." This information helps healthcare professionals quickly identify the contents of the blood bag.
Key Considerations:
- The product name must be clearly visible and easily readable.
- The description must be concise and accurate.
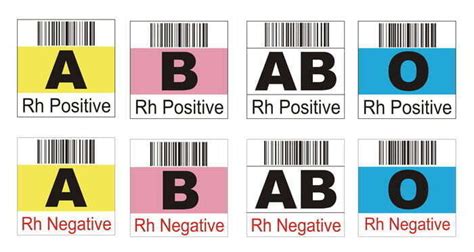
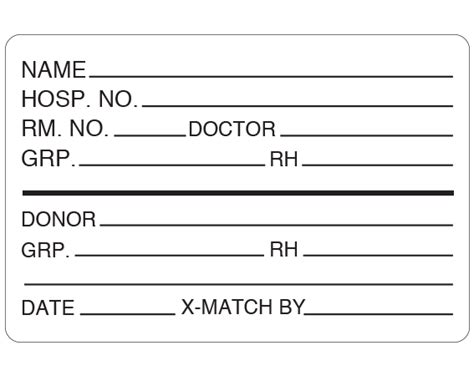
2. Blood Group and Type
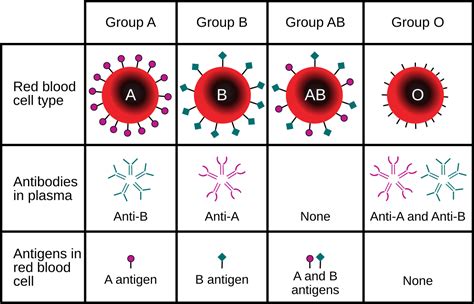
The blood group and type section must include the ABO blood group and Rh type of the donor. This information is critical in ensuring compatibility between the donor and recipient.
Key Considerations:
- The blood group and type must be clearly indicated (e.g., "A positive" or "O negative").
- The Rh type must be included (e.g., "Rh positive" or "Rh negative").
3. Donor Information

The donor information section must include the donor's identification number, name, and date of birth. This information helps track the donor's medical history and ensures that the blood product is linked to the correct donor.
Key Considerations:
- The donor's identification number must be unique and accurately recorded.
- The donor's name and date of birth must be clearly indicated.
4. Collection Date and Time
The collection date and time section must include the date and time the blood was collected from the donor. This information helps track the age of the blood product and ensures that it is used within the recommended timeframe.
Key Considerations:
- The collection date and time must be accurately recorded in a 24-hour format.
- The date must include the month, day, and year.
5. Expiration Date and Time

The expiration date and time section must include the date and time the blood product expires. This information ensures that the blood product is used before it becomes outdated and potentially unsafe.
Key Considerations:
- The expiration date and time must be accurately calculated based on the collection date and time.
- The date must include the month, day, and year.
6. Storage Conditions
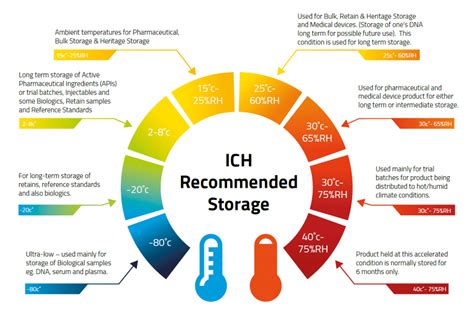
The storage conditions section must include information on the recommended storage conditions for the blood product, such as temperature and humidity levels. This information ensures that the blood product is stored correctly and maintains its potency.
Key Considerations:
- The storage conditions must be clearly indicated (e.g., "Store at 2-6°C" or "Store in a cool, dry place").
- The recommended storage duration must be included (e.g., "Store for up to 3 days").
7. Labeling and Certification Information

The labeling and certification information section must include information on the labeling and certification process, such as the name and address of the manufacturer and the certification number. This information ensures that the blood product meets regulatory requirements and is safe for use.
Key Considerations:
- The labeling and certification information must be clearly indicated (e.g., "Manufactured by XYZ Corporation" or "Certified by ABC Agency").
- The certification number must be accurately recorded.
Blood Bag Label Template Image Gallery
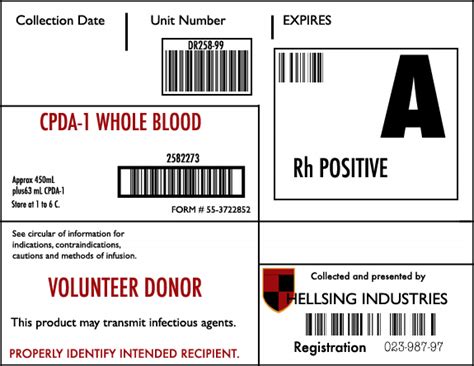



Conclusion and Call to Action
A well-designed blood bag label template is crucial in ensuring accurate identification and communication of blood products. By including the 7 essential elements outlined in this article, healthcare professionals can reduce the risk of errors and ensure patient safety. We encourage you to share your thoughts on the importance of blood bag labeling and how you use label templates in your daily practice. Leave a comment below or share this article with your colleagues to promote best practices in blood transfusion labeling.
