Intro
Discover endothermic reactions, where heat absorbs, and learn about thermodynamics, energy transfer, and chemical processes in this explanatory guide.
The world of chemistry is vast and fascinating, with various types of reactions that help us understand the intricacies of the physical world. One such concept is endothermic reactions, which play a crucial role in shaping our understanding of energy and its interactions with matter. Endothermic reactions are a fundamental aspect of chemistry, and their significance extends beyond the realm of academic curiosity, influencing various aspects of our daily lives. In this article, we will delve into the world of endothermic reactions, exploring their definition, characteristics, examples, and importance.
The concept of endothermic reactions is closely tied to the idea of energy and its role in chemical processes. Energy is the driving force behind all chemical reactions, and it can be either absorbed or released during a reaction. Endothermic reactions are characterized by the absorption of energy, typically in the form of heat, which is used to initiate and sustain the reaction. This absorption of energy leads to an increase in the internal energy of the reactants, resulting in the formation of products. The significance of endothermic reactions lies in their ability to demonstrate the complex interplay between energy and matter, highlighting the dynamic nature of chemical processes.
As we explore the world of endothermic reactions, it becomes clear that they are an integral part of various natural and industrial processes. From the decomposition of rocks to the manufacture of chemicals, endothermic reactions play a vital role in shaping our environment and driving technological advancements. The study of endothermic reactions has far-reaching implications, influencing fields such as materials science, environmental science, and engineering. By understanding the principles governing endothermic reactions, scientists and engineers can develop innovative solutions to real-world problems, driving progress and innovation.
What are Endothermic Reactions?
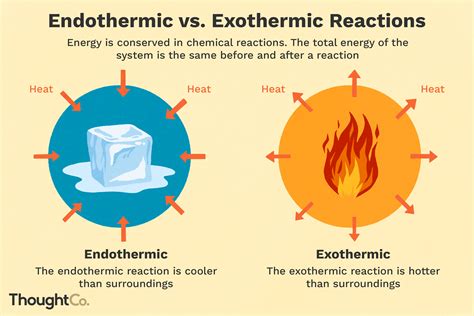
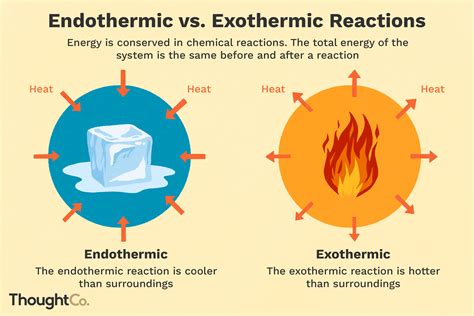
Endothermic reactions are chemical reactions that absorb energy, typically in the form of heat, from the surroundings. This energy is used to initiate and sustain the reaction, resulting in the formation of products. The term "endothermic" comes from the Greek words "endo," meaning "within," and "thermic," meaning "heat." Endothermic reactions are characterized by an increase in the internal energy of the reactants, which is reflected in the temperature change of the surroundings. During an endothermic reaction, the temperature of the surroundings decreases, indicating that energy is being absorbed from the environment.
Characteristics of Endothermic Reactions
Endothermic reactions exhibit several distinct characteristics that set them apart from other types of chemical reactions. Some of the key characteristics of endothermic reactions include: * Absorption of energy: Endothermic reactions absorb energy from the surroundings, which is used to initiate and sustain the reaction. * Increase in internal energy: The absorption of energy leads to an increase in the internal energy of the reactants, resulting in the formation of products. * Temperature decrease: The temperature of the surroundings decreases during an endothermic reaction, indicating that energy is being absorbed from the environment. * Spontaneity: Endothermic reactions are often non-spontaneous, meaning that they require an external energy source to proceed.Examples of Endothermic Reactions
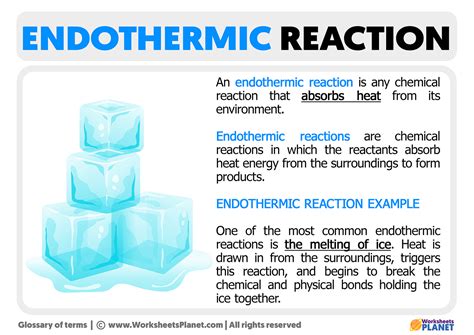
Endothermic reactions are ubiquitous in nature and industry, playing a crucial role in various processes. Some examples of endothermic reactions include:
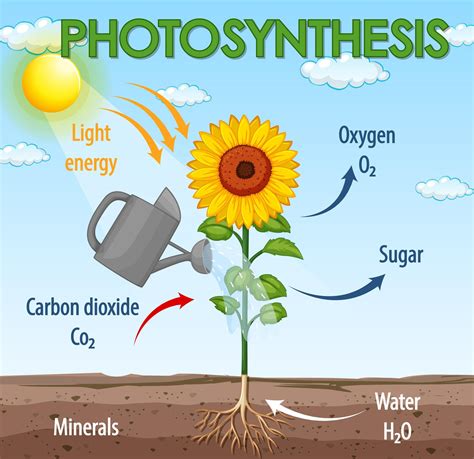
- Photosynthesis: The process by which plants convert carbon dioxide and water into glucose and oxygen, absorbing energy from sunlight.
- Decomposition of rocks: The breakdown of rocks into simpler minerals, absorbing energy from the surroundings.
- Manufacture of chemicals: Many industrial processes, such as the production of ammonia and methanol, involve endothermic reactions.
- Melting of ice: The absorption of energy from the surroundings to change the state of water from solid to liquid.
Importance of Endothermic Reactions
The importance of endothermic reactions extends beyond the realm of academic curiosity, influencing various aspects of our daily lives. Some of the key reasons why endothermic reactions are important include: * Understanding energy interactions: Endothermic reactions help us understand the complex interplay between energy and matter, highlighting the dynamic nature of chemical processes. * Industrial applications: Endothermic reactions are used in various industrial processes, such as the manufacture of chemicals and the production of energy. * Environmental significance: Endothermic reactions play a crucial role in shaping our environment, influencing processes such as photosynthesis and the decomposition of rocks.Working Mechanisms of Endothermic Reactions
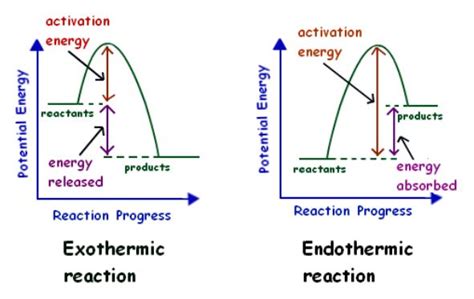
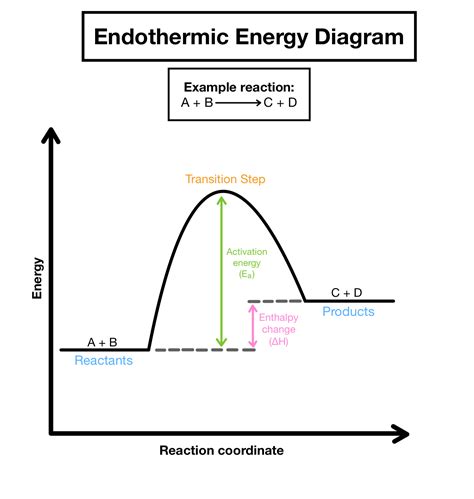
The working mechanisms of endothermic reactions involve the absorption of energy from the surroundings, which is used to initiate and sustain the reaction. The energy is typically absorbed in the form of heat, which increases the internal energy of the reactants. This increase in internal energy leads to the formation of products, which can be either stable or unstable. The stability of the products depends on the energy change associated with the reaction, as well as the conditions under which the reaction occurs.
Steps Involved in Endothermic Reactions
The steps involved in endothermic reactions include: 1. Absorption of energy: The reaction absorbs energy from the surroundings, which is used to initiate and sustain the reaction. 2. Increase in internal energy: The absorption of energy leads to an increase in the internal energy of the reactants. 3. Formation of products: The increase in internal energy leads to the formation of products, which can be either stable or unstable. 4. Energy change: The energy change associated with the reaction determines the stability of the products and the conditions under which the reaction occurs.Practical Applications of Endothermic Reactions
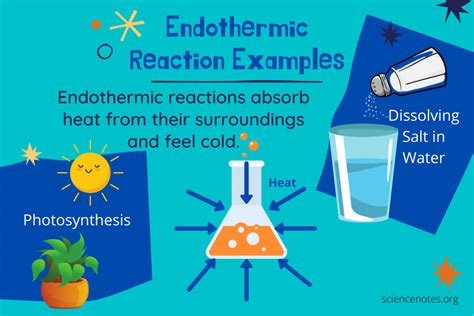
The practical applications of endothermic reactions are diverse and widespread, influencing various aspects of our daily lives. Some of the key applications of endothermic reactions include:
- Industrial processes: Endothermic reactions are used in various industrial processes, such as the manufacture of chemicals and the production of energy.
- Environmental management: Endothermic reactions play a crucial role in shaping our environment, influencing processes such as photosynthesis and the decomposition of rocks.
- Energy production: Endothermic reactions can be used to produce energy, such as in the case of nuclear power plants.
Benefits and Challenges of Endothermic Reactions
The benefits and challenges of endothermic reactions are closely tied to their practical applications. Some of the key benefits of endothermic reactions include: * Energy production: Endothermic reactions can be used to produce energy, such as in the case of nuclear power plants. * Industrial applications: Endothermic reactions are used in various industrial processes, such as the manufacture of chemicals and the production of energy. * Environmental significance: Endothermic reactions play a crucial role in shaping our environment, influencing processes such as photosynthesis and the decomposition of rocks.However, endothermic reactions also pose several challenges, including:
- Energy requirements: Endothermic reactions require an external energy source to proceed, which can be a limitation in certain situations.
- Stability of products: The stability of the products formed during an endothermic reaction can be a concern, as unstable products can pose safety risks.
- Environmental impact: The environmental impact of endothermic reactions can be significant, as they can influence processes such as climate change and pollution.
Gallery of Endothermic Reactions
Endothermic Reactions Image Gallery






As we conclude our exploration of endothermic reactions, it becomes clear that these processes play a vital role in shaping our understanding of energy and its interactions with matter. The importance of endothermic reactions extends beyond the realm of academic curiosity, influencing various aspects of our daily lives. We invite you to share your thoughts and insights on the significance of endothermic reactions, and how they can be applied to real-world problems. Whether you are a student, researcher, or simply someone interested in learning more about the world of chemistry, we encourage you to engage with this topic and explore its many facets. By working together, we can deepen our understanding of endothermic reactions and harness their potential to drive innovation and progress.
