Intro
Master FDA pre-submission templates with ease. Discover 5 essential tips to streamline your pre-submission process, ensuring compliance with FDA regulations. Learn how to create a winning pre-submission package, avoiding common pitfalls and reducing review timelines. Expert insights on pre-submission template best practices, regulatory compliance, and more.
The FDA pre-submission process is a critical step in the development of medical devices, drugs, and biologics. It allows sponsors to engage with the FDA to discuss their product development plans and receive feedback on their submission strategy. A well-structured pre-submission template is essential to ensure a successful meeting with the FDA. Here are five essential FDA pre-submission template tips to help you prepare for a productive meeting.
Tip 1: Clearly Define the Purpose and Scope of the Meeting

When preparing your pre-submission template, it's crucial to clearly define the purpose and scope of the meeting. This will help you stay focused on the key topics to discuss with the FDA and ensure that you receive the feedback you need. The purpose of the meeting should be concise and specific, outlining the specific issues or questions you want to address. The scope of the meeting should also be clearly defined, including the specific topics to be discussed and the expected outcomes.
Benefits of Clearly Defining the Purpose and Scope of the Meeting
- Ensures that the meeting stays focused on the key topics
- Helps the FDA understand the sponsor's needs and expectations
- Allows for more productive and efficient discussions
- Helps to manage the time and agenda of the meeting
Tip 2: Provide a Comprehensive Product Overview
A comprehensive product overview is essential to help the FDA understand the sponsor's product and its intended use. This section should include a detailed description of the product, its indications for use, and its technical specifications. The product overview should also include any relevant information about the product's development, including any previous meetings or correspondence with the FDA.
Key Components of a Comprehensive Product Overview
- Detailed product description
- Indications for use
- Technical specifications
- Previous meetings or correspondence with the FDA
- Product development history
Tip 3: Clearly Outline the Sponsor's Questions and Objectives
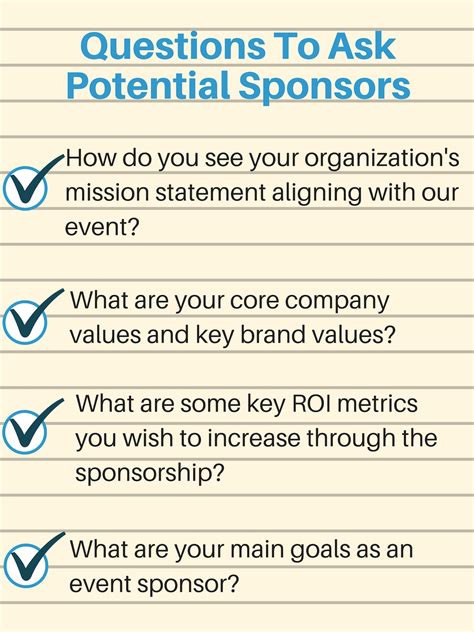
The sponsor's questions and objectives are a critical component of the pre-submission template. This section should clearly outline the specific questions and concerns that the sponsor has regarding the product's development and submission strategy. The sponsor's objectives should also be clearly stated, including any specific feedback or guidance that is being sought from the FDA.
Benefits of Clearly Outlining the Sponsor's Questions and Objectives
- Ensures that the FDA understands the sponsor's needs and expectations
- Helps to focus the discussion on the key topics
- Allows for more productive and efficient discussions
- Helps to ensure that the sponsor receives the feedback and guidance they need
Tip 4: Provide Supporting Documentation and Data

The pre-submission template should include any supporting documentation and data that is relevant to the sponsor's questions and objectives. This may include data from preclinical studies, clinical trials, or other sources. The documentation and data should be clearly organized and easy to review, with concise summaries and explanations.
Key Components of Supporting Documentation and Data
- Data from preclinical studies
- Data from clinical trials
- Other relevant data and documentation
- Concise summaries and explanations
Tip 5: Ensure Compliance with FDA Regulations and Guidance

The pre-submission template should be carefully reviewed to ensure compliance with FDA regulations and guidance. This includes ensuring that the template is complete and accurate, and that all relevant information is included. The sponsor should also be familiar with the FDA's policies and procedures regarding pre-submission meetings, and should ensure that the template is consistent with these policies.
Benefits of Ensuring Compliance with FDA Regulations and Guidance
- Helps to ensure that the sponsor is compliant with FDA regulations and guidance
- Reduces the risk of delays or rejection of the submission
- Helps to build trust and credibility with the FDA
- Ensures that the sponsor receives accurate and reliable feedback from the FDA
FDA Pre-Submission Template Image Gallery






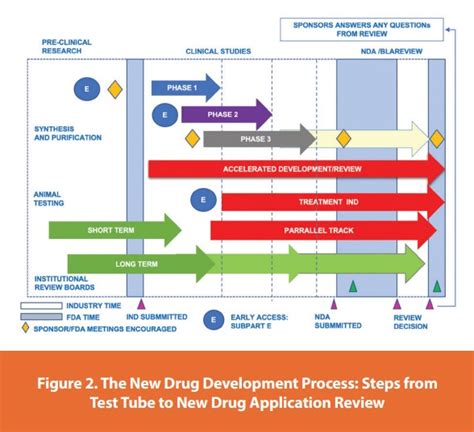

By following these five essential FDA pre-submission template tips, sponsors can ensure that they are well-prepared for a productive meeting with the FDA. A clear and comprehensive pre-submission template is critical to ensuring that the sponsor receives the feedback and guidance they need to successfully develop and submit their product.
