Intro
Boost medical device quality with ISO 13485 software validation. Learn the 5 essential steps to ensure compliance, from risk management to verification and validation. Streamline your QMS, reduce errors, and increase patient safety with our expert guide on ISO 13485 software validation, regulatory compliance, and medical device software development.
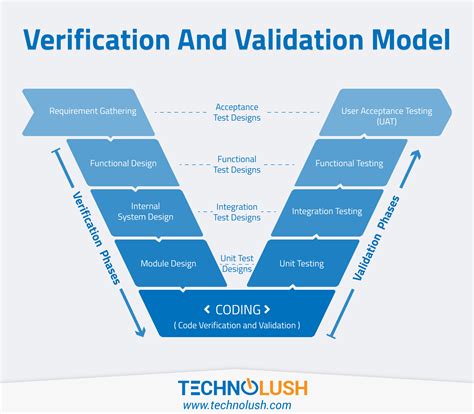
In the medical device industry, software validation is a critical process that ensures the safety and effectiveness of medical devices that rely on software. ISO 13485, a quality management system standard for medical devices, emphasizes the importance of software validation in its clause 4.1.6. In this article, we will discuss the 5 steps to ISO 13485 software validation.
Medical devices that rely on software, such as insulin pumps, pacemakers, and MRI machines, require software validation to ensure that they perform their intended functions safely and effectively. Software validation is a systematic process that evaluates the software's ability to meet its specifications and regulatory requirements. It involves a series of tests and evaluations that verify the software's functionality, performance, and safety.
What is ISO 13485 Software Validation?
ISO 13485 software validation is a process that ensures the software used in medical devices meets the requirements of the ISO 13485 standard. The standard requires medical device manufacturers to establish a quality management system that ensures the safety and effectiveness of their products. Software validation is an essential part of this quality management system.
ISO 13485 software validation involves a series of activities that evaluate the software's ability to meet its specifications and regulatory requirements. These activities include software design, development, testing, and deployment. The goal of software validation is to ensure that the software performs its intended functions safely and effectively.
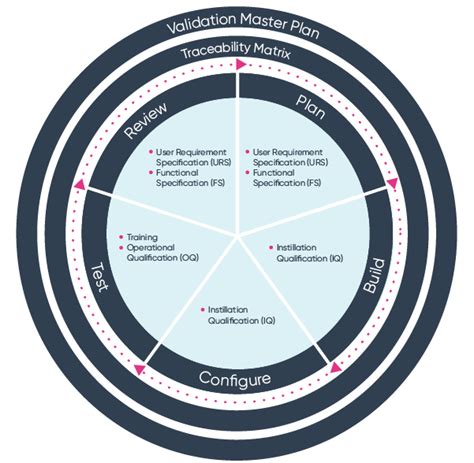
Step 1: Software Design and Development
The first step in ISO 13485 software validation is software design and development. During this phase, the software development team creates the software design specifications, develops the software code, and conducts unit testing and integration testing. The software design specifications should include the software's functional and performance requirements, as well as its safety and security requirements.
The software development team should follow a structured design and development process that includes:
- Requirements gathering and analysis
- Software design and architecture
- Software coding and testing
- Code reviews and walk-throughs
- Unit testing and integration testing

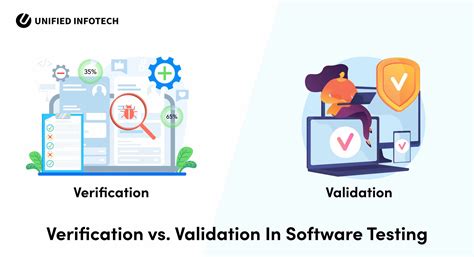
Step 2: Software Verification and Validation
The second step in ISO 13485 software validation is software verification and validation. During this phase, the software development team verifies and validates the software against its design specifications and regulatory requirements. Verification involves evaluating the software's functionality and performance, while validation involves evaluating the software's safety and effectiveness.
The software development team should conduct a series of tests and evaluations that include:
- Functional testing
- Performance testing
- Safety testing
- Security testing
- Usability testing
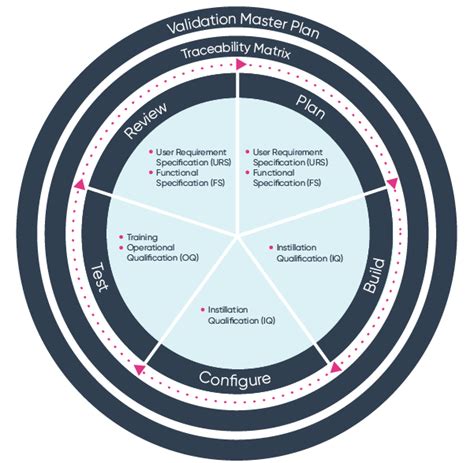
Step 3: Software Deployment and Installation
The third step in ISO 13485 software validation is software deployment and installation. During this phase, the software development team deploys and installs the software on the medical device. The software development team should ensure that the software is properly configured and installed on the medical device.
The software development team should conduct a series of tests and evaluations that include:
- Installation testing
- Configuration testing
- Compatibility testing

Step 4: Software Maintenance and Updates
The fourth step in ISO 13485 software validation is software maintenance and updates. During this phase, the software development team maintains and updates the software to ensure that it remains safe and effective. The software development team should have a plan in place for software maintenance and updates that includes:
- Software updates and patches
- Software bug fixes
- Software enhancements

Step 5: Software Retirement and Disposal
The fifth step in ISO 13485 software validation is software retirement and disposal. During this phase, the software development team retires and disposes of the software when it is no longer needed or supported. The software development team should have a plan in place for software retirement and disposal that includes:
- Software retirement planning
- Software disposal procedures

In conclusion, ISO 13485 software validation is a critical process that ensures the safety and effectiveness of medical devices that rely on software. The 5 steps to ISO 13485 software validation include software design and development, software verification and validation, software deployment and installation, software maintenance and updates, and software retirement and disposal. By following these steps, medical device manufacturers can ensure that their software meets the requirements of the ISO 13485 standard and is safe and effective for use.
Gallery of ISO 13485 Software Validation Images
ISO 13485 Software Validation Image Gallery





We hope this article has provided you with a comprehensive understanding of the 5 steps to ISO 13485 software validation. If you have any questions or need further clarification, please do not hesitate to comment below.
