Unlock the secrets of atmospheric composition with the molar mass of air. Discover how this fundamental constant influences air density, molecular interactions, and Earths climate. Explore the intricacies of atmospheric science, including gas mixture calculations, density variations, and the impacts of temperature and pressure on airs molar mass.
The concept of molar mass is a fundamental principle in chemistry, allowing us to understand the composition of various substances. When it comes to air, the molar mass of its components plays a crucial role in determining its overall properties and behavior. In this article, we will delve into the world of atmospheric composition and explore the significance of molar mass in understanding the air we breathe.
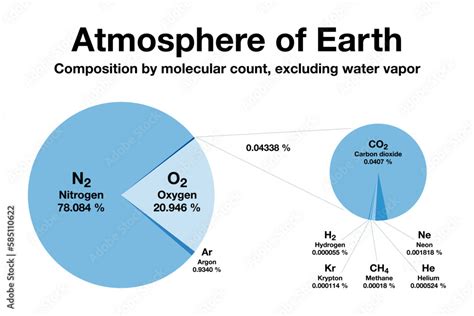
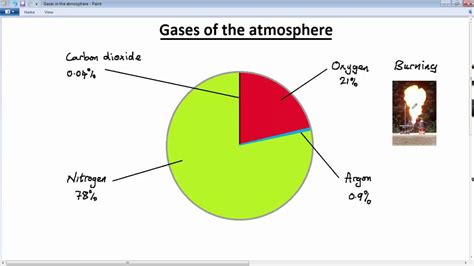
The air we inhale is a complex mixture of gases, primarily consisting of nitrogen (N2), oxygen (O2), argon (Ar), and trace amounts of other gases. The molar mass of air is a weighted average of the molar masses of its individual components. By calculating the molar mass of air, we can gain valuable insights into its composition and behavior.
Understanding Molar Mass
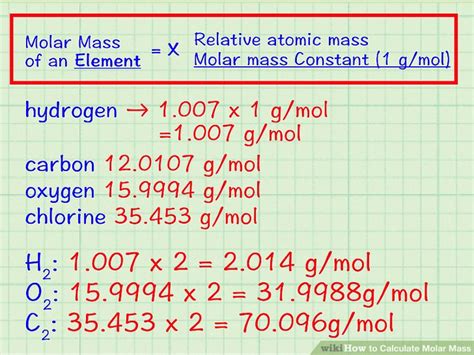
Molar mass is a measure of the mass of one mole of a substance, typically expressed in units of grams per mole (g/mol). It is a fundamental property of a substance, allowing us to understand its composition and behavior. In the context of air, the molar mass of its components plays a crucial role in determining its overall properties.
Calculating Molar Mass
To calculate the molar mass of air, we need to know the molar masses of its individual components. The most abundant gases in air are nitrogen (N2), oxygen (O2), and argon (Ar), with molar masses of 28.01 g/mol, 32.00 g/mol, and 39.95 g/mol, respectively. We can calculate the molar mass of air by taking a weighted average of these values, based on their relative abundance.
Importance of Molar Mass in Atmospheric Composition
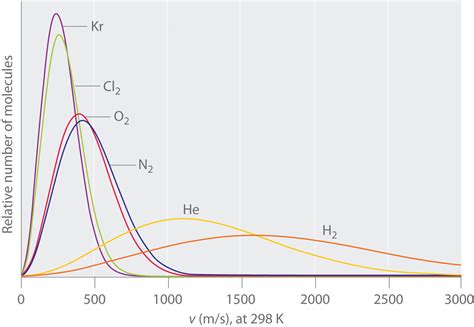
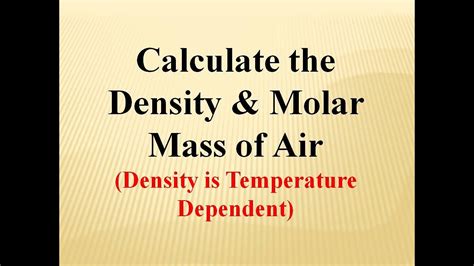
The molar mass of air is crucial in understanding its composition and behavior. It affects the density of air, which in turn influences its buoyancy and ability to support life. The molar mass of air also plays a role in determining its thermal properties, such as its specific heat capacity and thermal conductivity.
Applications of Molar Mass in Atmospheric Science
The molar mass of air has numerous applications in atmospheric science, including:
- Understanding weather patterns and climate change
- Predicting air quality and pollution levels
- Modeling atmospheric circulation and transport of pollutants
- Studying the properties of aerosols and clouds

Molar Mass of Air Components
The molar masses of air components are essential in calculating the overall molar mass of air. Here are the molar masses of some of the most abundant gases in air:
- Nitrogen (N2): 28.01 g/mol
- Oxygen (O2): 32.00 g/mol
- Argon (Ar): 39.95 g/mol
- Carbon dioxide (CO2): 44.01 g/mol
- Water vapor (H2O): 18.02 g/mol

Variations in Molar Mass of Air
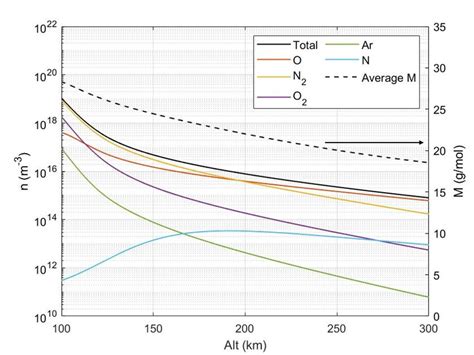
The molar mass of air can vary depending on several factors, including:
- Altitude: The molar mass of air decreases with increasing altitude due to the decrease in air pressure.
- Temperature: The molar mass of air increases with increasing temperature due to the expansion of air molecules.
- Humidity: The molar mass of air increases with increasing humidity due to the addition of water vapor molecules.
Conclusion and Future Directions
In conclusion, the molar mass of air is a fundamental property that plays a crucial role in understanding its composition and behavior. By calculating the molar mass of air, we can gain valuable insights into its properties and behavior. Future research directions include studying the variations in molar mass of air due to different environmental factors and exploring its applications in atmospheric science.
Molar Mass of Air Image Gallery

We hope this article has provided you with a comprehensive understanding of the molar mass of air and its significance in atmospheric composition. If you have any questions or comments, please feel free to share them below.
