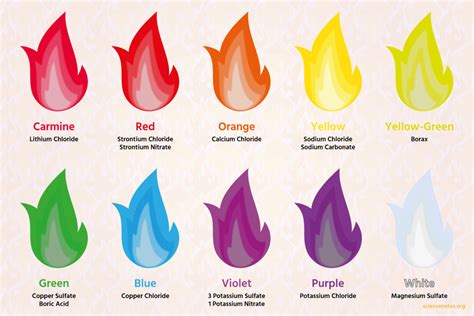
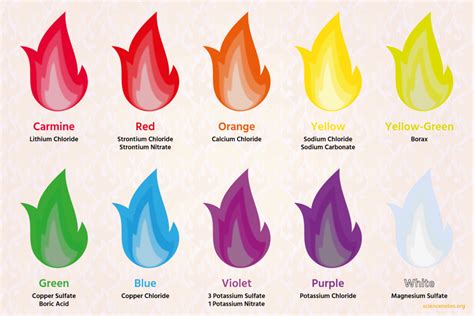
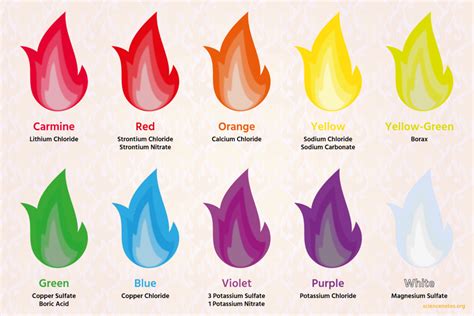
Unlock the secrets of the 5 colors of fire, a phenomenon that reveals the presence of elements like copper, lithium, and sodium. Learn how to identify the distinct hues, from red to violet, and their corresponding temperatures, to gain a deeper understanding of fire chemistry and the art of pyrotechnics.
Fire has been a vital element for human survival and development, providing warmth, light, and a means of cooking food. While we often associate fire with its typical orange-yellow color, it can actually appear in a range of colors depending on the temperature and chemical composition of the flames. In this article, we'll delve into the five colors of fire, exploring the science behind each hue and the conditions that produce them.
Understanding the Science of Fire Colors

Fire is a chemical reaction that involves the rapid oxidation of fuel sources, releasing energy in the form of heat and light. The color of fire is determined by the temperature of the flames and the presence of certain chemicals. When a fuel source is heated, it releases energy in the form of photons, which our eyes perceive as light. The color of the light depends on the energy level of the photons, with higher-energy photons producing shorter wavelengths of light, such as blue and violet, while lower-energy photons produce longer wavelengths, like red and orange.
The Temperature of Fire Colors
The temperature of fire is a critical factor in determining its color. As the temperature of the flames increases, the color of the fire changes. Here's a rough guide to the temperature ranges associated with different fire colors:
- Red: 500-800°C (932-1,472°F)
- Orange: 800-1,000°C (1,472-1,832°F)
- Yellow: 1,000-1,300°C (1,832-2,372°F)
- White: 1,300-1,800°C (2,372-3,272°F)
- Blue: 1,800-2,000°C (3,272-3,632°F)
The Five Colors of Fire
Now that we've covered the basics of fire colors, let's dive into the five main colors of fire, exploring the conditions that produce each hue.
1. Red Fire
Red fire is typically produced by lower-temperature flames, often seen in smoldering fires or those fueled by organic materials like wood or coal. The red color is due to the presence of incandescent particles, such as soot or ash, which emit light at longer wavelengths.
2. Orange Fire
Orange fire is produced by moderate-temperature flames, often seen in fires fueled by materials like paper or cardboard. The orange color is due to the presence of excited particles, such as sodium or calcium, which emit light at specific wavelengths.
3. Yellow Fire
Yellow fire is produced by higher-temperature flames, often seen in fires fueled by materials like gasoline or propane. The yellow color is due to the presence of excited particles, such as hydrogen or helium, which emit light at specific wavelengths.
4. White Fire
White fire is produced by extremely high-temperature flames, often seen in fires fueled by materials like hydrogen or oxygen. The white color is due to the presence of incandescent particles, such as soot or ash, which emit light at all wavelengths.
5. Blue Fire
Blue fire is produced by extremely high-temperature flames, often seen in fires fueled by materials like methane or ethane. The blue color is due to the presence of excited particles, such as copper or silver, which emit light at specific wavelengths.
Practical Applications of Fire Colors
Understanding the colors of fire has practical applications in various fields, including:
- Firefighting: Knowing the color of a fire can help firefighters determine the temperature and composition of the flames, which can inform their extinguishing strategy.
- Materials science: Studying the colors of fire can help researchers develop new materials with unique properties, such as high-temperature resistance.
- Art and design: Fire colors can be used in art and design to create striking visual effects, such as in fire dancing or pyrotechnic displays.
Conclusion and Final Thoughts
In conclusion, the colors of fire are a fascinating phenomenon that can provide insights into the chemistry and physics of combustion. By understanding the science behind fire colors, we can develop new technologies, improve our understanding of materials, and appreciate the beauty of fire in all its forms.

Gallery of Fire Colors
Fire Colors Image Gallery