Intro
Develop a comprehensive ISO 17025 quality manual template in 8 straightforward steps. Learn how to create a robust quality management system, define scope and responsibilities, establish policies and procedures, and ensure compliance with the standard. Enhance your laboratorys credibility and efficiency with this structured approach to ISO 17025 implementation.
Creating an ISO 17025 quality manual template is a crucial step for laboratories seeking to achieve accreditation and maintain the highest standards of quality management. The ISO 17025 standard is a globally recognized benchmark for laboratories, emphasizing the importance of a well-structured quality management system. A quality manual serves as the backbone of this system, outlining policies, procedures, and responsibilities that ensure the laboratory's operations meet the standard's requirements. Here's a step-by-step guide to creating an effective ISO 17025 quality manual template:

Step 1: Define the Scope and Structure
The first step in creating an ISO 17025 quality manual template is to define the scope and structure of the document. This involves identifying the laboratory's activities, services, and areas that the quality manual will cover. The structure should be organized in a logical manner, making it easy for readers to navigate through the document.
Step 2: Establish the Quality Policy
The quality policy is a crucial element of the quality manual, as it outlines the laboratory's commitment to quality and its objectives. The policy should be concise, clear, and communicated to all personnel. It forms the foundation of the quality management system and should be regularly reviewed and updated.
Step 3: Document Procedures and Processes
This step involves documenting all procedures and processes that are critical to the laboratory's operations. These include sampling, testing, calibration, and reporting procedures, as well as processes for quality control, internal audits, and management reviews. Each procedure should be detailed, including responsibilities, equipment, and records to be maintained.
Step 4: Define Roles and Responsibilities
Clearly defining roles and responsibilities within the laboratory is essential for the effective implementation of the quality management system. This includes the roles of the quality manager, technical manager, and other key personnel. Job descriptions should be detailed, outlining specific duties, authorities, and responsibilities.
Step 5: Outline Quality Control Measures
The quality manual should outline the quality control measures the laboratory has in place to ensure the accuracy and reliability of its results. This includes procedures for calibration and maintenance of equipment, quality control checks, and corrective actions to be taken in case of deviations.
Step 6: Establish a Document Control System
A document control system is necessary to ensure that all documents, including the quality manual, procedures, and records, are properly controlled and maintained. This involves establishing a system for document approval, issue, and revision, as well as ensuring that obsolete documents are removed from circulation.
Step 7: Address Internal Audits and Management Reviews
Internal audits and management reviews are critical components of the ISO 17025 standard. The quality manual should outline the procedures for conducting internal audits, including the frequency, scope, and responsibilities. It should also describe the process for management reviews, including the inputs, outputs, and actions arising from the reviews.
Step 8: Review and Revise the Quality Manual
The final step is to review and revise the quality manual template to ensure it meets the requirements of the ISO 17025 standard and is relevant to the laboratory's operations. This involves seeking input from all personnel and stakeholders, and making any necessary updates or changes.

Gallery of ISO 17025 Quality Manual Templates:
ISO 17025 Quality Manual Templates






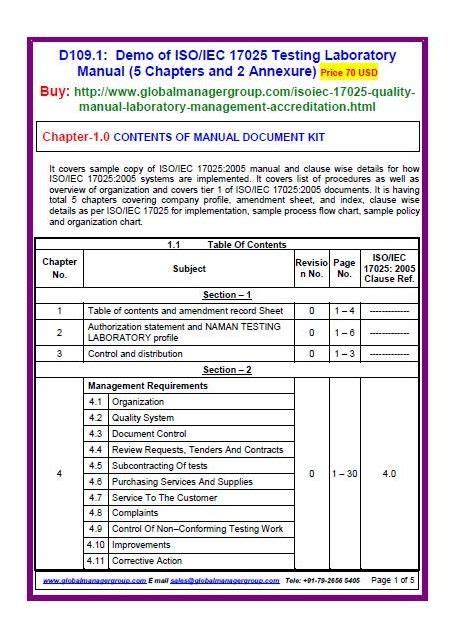



By following these steps and using the provided gallery of ISO 17025 quality manual templates as a reference, laboratories can create a comprehensive and effective quality manual that meets the requirements of the ISO 17025 standard and supports their accreditation and quality management goals.
