Intro
Discover Halite mineral properties, including its crystal structure, chemical composition, and geological occurrences, with related terms like rock salt, mineralogy, and geological formations.
Halite, commonly known as rock salt, is a naturally occurring mineral composed of sodium chloride (NaCl). It is one of the most abundant minerals found on Earth, with vast deposits located all over the world. Halite has been a vital resource throughout human history, serving not only as a seasoning for food but also playing a significant role in various industrial and economic applications. The unique properties of halite make it an essential mineral in our daily lives, and understanding its characteristics is crucial for appreciating its importance.
The formation of halite occurs through the evaporation of seawater or other salt-rich water bodies. As the water evaporates, it leaves behind a concentration of salts, including sodium chloride, which then crystallizes into halite. This process can happen in a variety of environments, such as salt lakes, salt springs, and marine environments. The resulting halite deposits can range from small, shallow accumulations to large, deep formations that stretch over vast areas. The economic extraction of halite is often associated with these large deposits, which can be mined using conventional mining techniques or through solution mining, where water is injected into the deposit to dissolve the salt, which is then pumped out and evaporated to recover the salt.
Halite's mineral properties are what make it so valuable and versatile. It has a distinctive cubic crystal structure, which gives it a characteristic taste and allows it to cleave easily along its crystal planes. This cleavage property is one reason why halite is often used in cooking and as a preservative, as it can be easily crushed or ground into smaller particles. Additionally, halite has a relatively low melting point compared to other minerals, which makes it useful in applications where a substance with a specific melting behavior is required. The optical properties of halite are also noteworthy, as it can exhibit a range of colors when pure, from colorless and transparent to white, gray, or even colored due to impurities.
Physical Properties of Halite

The physical properties of halite are crucial for its identification and use. Halite has a hardness of 2.5 on the Mohs scale, which makes it a relatively soft mineral. It is also highly soluble in water, which is why it is often found in areas where water has evaporated, leaving behind salt deposits. The density of halite is approximately 2.17 g/cm³, which is slightly above that of water, causing it to sink in aqueous solutions. Its cubic crystal structure, as mentioned, is one of its most distinctive features, both visually and in terms of its physical behavior.
Chemical Composition and Reactions
The chemical composition of halite is straightforward, consisting of sodium (Na) and chlorine (Cl) ions in a 1:1 ratio, forming sodium chloride (NaCl). This composition is stable under normal conditions but can react with certain substances. For example, when halite is exposed to sulfuric acid, it can react to form hydrochloric acid and sodium sulfate. Understanding these chemical reactions is important for various industrial processes that utilize halite.Economic Importance of Halite

The economic importance of halite cannot be overstated. It is a critical component in the chemical industry, where it is used to produce chlorine and sodium hydroxide through the electrolysis of sodium chloride solutions. These products are then used in the manufacture of plastics, dyes, and pharmaceuticals, among other things. Halite is also essential in the preservation of food, particularly meat, where it acts as a preservative by drawing out moisture and creating an environment inhospitable to bacterial growth. Additionally, halite is used to de-ice roads in cold climates, as it lowers the freezing point of water when applied to ice and snow, thus facilitating traffic flow during winter months.
Environmental Impact and Sustainability
While halite is a naturally occurring substance, its extraction and use can have environmental implications. Large-scale mining operations can disrupt local ecosystems, and the use of halite for de-icing can lead to increased salt concentrations in water bodies, potentially harming aquatic life. Therefore, it is essential to manage halite resources sustainably, ensuring that extraction methods minimize environmental damage and that its use is optimized to reduce waste and mitigate adverse effects on the environment.Halite in Everyday Life

Halite's presence in everyday life is ubiquitous. Beyond its use as a seasoning in food, halite is found in a variety of consumer products, from cosmetics to pharmaceuticals. Its preservative qualities make it an essential ingredient in the food industry, and its role in maintaining healthy skin and preventing infection is well-documented. Furthermore, the de-icing properties of halite are crucial for maintaining safe road conditions during winter, reducing the risk of accidents caused by slippery surfaces.
Cultural and Historical Significance
The cultural and historical significance of halite extends beyond its practical uses. In many societies, salt has been a symbol of wealth, prosperity, and preservation. The Roman Empire, for example, used salt as a form of currency in some transactions, and the word "salary" is derived from the Latin word for salt, "salarium." In various cultures, salt is also used in rituals and ceremonies, often symbolizing purification, protection, or good fortune.Health Benefits and Risks

Halite, as a source of sodium, is essential for human health in small quantities. Sodium helps regulate the amount of water in the body and facilitates the transmission of nerve impulses. However, excessive consumption of halite can lead to health issues, including high blood pressure, heart disease, and stroke. It is crucial to balance the intake of halite with other nutrients to maintain a healthy diet.
Nutritional Value and Dietary Recommendations
The nutritional value of halite is primarily attributed to its sodium content. While it does not provide any calories, halite is vital for maintaining proper bodily functions. Dietary recommendations typically advise limiting sodium intake to less than 2,300 milligrams per day, with further reduction to 1,500 milligrams per day for those who are at risk for high blood pressure or heart disease. Understanding these guidelines is essential for making informed choices about halite consumption.Industrial Applications of Halite

The industrial applications of halite are diverse and extensive. In addition to its use in the chemical industry for the production of chlorine and sodium hydroxide, halite is used in the manufacture of paper, dyes, and textiles. It is also an essential component in the production of soap and glass. The de-icing properties of halite make it crucial for winter road maintenance, and its use in oil and gas exploration helps to stabilize drilling fluids and improve the extraction process.
Future Prospects and Challenges
Looking to the future, the demand for halite is expected to continue, driven by its essential role in various industries and its use in everyday life. However, challenges such as environmental sustainability, efficient extraction methods, and balancing consumption with health recommendations will need to be addressed. Innovations in mining technology and the development of more sustainable practices will be key in ensuring that halite resources are managed responsibly.Gallery of Halite-Related Images
Halite Image Gallery

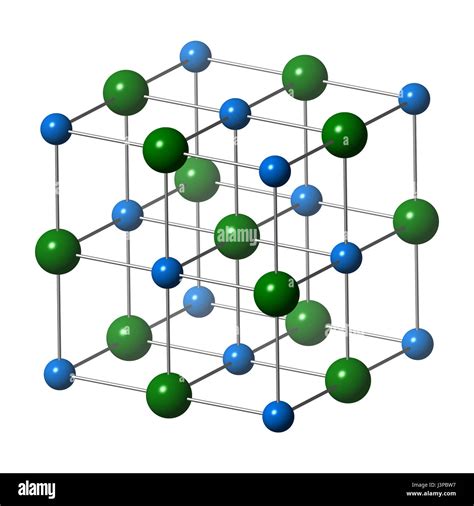






In conclusion, halite is a mineral with a wide range of applications, from everyday use as a seasoning to its critical role in various industries. Its unique properties, including its cubic crystal structure and high solubility in water, make it an essential resource. As we move forward, it is crucial to balance the demand for halite with sustainable practices and environmental considerations. We invite you to share your thoughts on the importance of halite and how it impacts your daily life. Whether through comments, shares, or further research, your engagement with this topic can help raise awareness about the significance of halite and the need for responsible management of this vital mineral resource.
