Intro
Discover 5 ways solvolysis reaction enhances chemical processes, including nucleophilic substitution, hydrolysis, and elimination reactions, with applications in organic chemistry and synthesis.
Solvolysis reactions are a fundamental concept in organic chemistry, playing a crucial role in understanding the mechanisms and outcomes of various chemical transformations. The term "solvolysis" refers to a type of reaction where a solvent participates in the cleavage of a chemical bond, often leading to the formation of new compounds. This phenomenon is not only significant in synthetic organic chemistry but also has implications in fields such as pharmaceuticals, materials science, and environmental chemistry.
The importance of solvolysis reactions stems from their ability to facilitate the breaking and forming of bonds in molecules, which is essential for synthesizing complex organic compounds. These reactions can occur under various conditions and with different types of solvents, influencing the reaction pathway and the products formed. Understanding the mechanisms and controlling factors of solvolysis reactions is vital for chemists to design and optimize synthetic routes efficiently.
Solvolysis reactions are particularly useful in the context of organic synthesis due to their versatility and the wide range of substrates they can act upon. By carefully selecting the solvent and reaction conditions, chemists can influence the reaction outcome, including the stereochemistry of the products. This level of control is crucial in the synthesis of complex molecules, where the correct stereochemical arrangement can significantly affect the biological activity or physical properties of the compound.
Introduction to Solvolysis Reactions
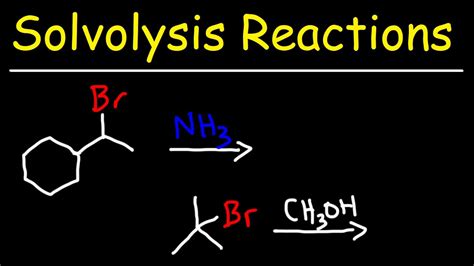
Solvolysis reactions involve the interaction of a solvent with a substrate, leading to the cleavage of a chemical bond. This process can be facilitated by various factors, including the polarity of the solvent, temperature, and the presence of catalysts. The solvent's role in solvolysis is not merely as an inert medium but as an active participant that can influence the reaction mechanism and the stability of intermediates.
Types of Solvolysis Reactions
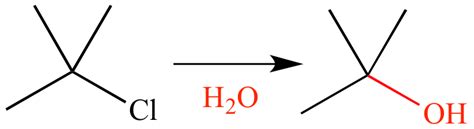
Solvolysis reactions can be categorized based on the type of bond being cleaved and the nature of the solvent involved. For instance, hydrolysis is a type of solvolysis where water acts as the solvent, leading to the cleavage of esters, amides, or other functional groups. Similarly, alcoholysis involves alcohols as solvents, and these reactions are crucial in the synthesis of esters and ethers.Mechanisms of Solvolysis Reactions
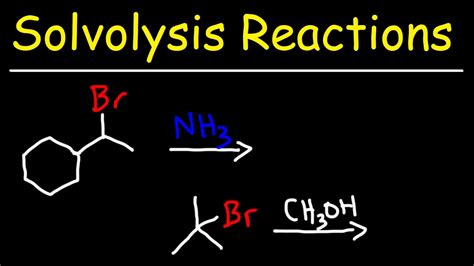
The mechanism of a solvolysis reaction is critical in understanding how the reaction proceeds and what factors influence its outcome. Generally, solvolysis reactions can occur via nucleophilic substitution mechanisms, where the solvent acts as a nucleophile attacking the substrate. The SN1 and SN2 mechanisms are two primary pathways for nucleophilic substitution, differing in their stereochemical outcomes and the role of the solvent in the rate-determining step.
Nucleophilic Substitution Mechanisms
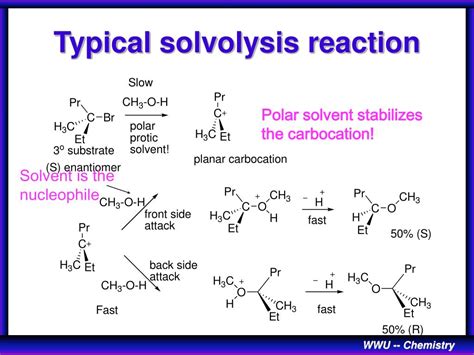
- **SN1 Mechanism**: This pathway involves a two-step process where the substrate first undergoes ionization to form a carbocation intermediate, followed by nucleophilic attack by the solvent. The SN1 mechanism is characterized by a first-order rate law and often leads to racemization of the product. - **SN2 Mechanism**: In contrast, the SN2 mechanism is a concerted process where the nucleophilic attack by the solvent and the departure of the leaving group occur simultaneously. This pathway is second-order and typically results in inversion of configuration at the reaction center.Factors Influencing Solvolysis Reactions

Several factors can influence the rate and outcome of solvolysis reactions, including the nature of the solvent, the structure of the substrate, temperature, and the presence of catalysts. Understanding these factors is crucial for optimizing solvolysis reactions in synthetic applications.
Solvent Effects
The choice of solvent is critical in solvolysis reactions, as it can significantly affect the reaction rate and the stability of intermediates. Polar protic solvents, such as water and alcohols, can participate in hydrogen bonding and are effective in stabilizing charged intermediates, thereby facilitating the reaction.Applications of Solvolysis Reactions
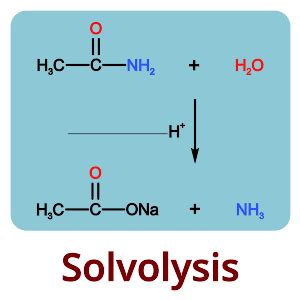
Solvolysis reactions have widespread applications in organic synthesis, pharmaceutical chemistry, and materials science. These reactions are utilized in the synthesis of complex molecules, including natural products, drugs, and polymers. The ability to control the stereochemistry and regiochemistry of solvolysis reactions makes them particularly valuable in the synthesis of biologically active compounds.
Pharmaceutical Applications
In pharmaceutical chemistry, solvolysis reactions are employed in the synthesis of active pharmaceutical ingredients (APIs). The synthesis of drugs often requires the formation of specific stereoisomers, which can be achieved through carefully controlled solvolysis reactions.Future Perspectives and Challenges

Despite the significant advancements in understanding and applying solvolysis reactions, there are still challenges to be addressed. The development of more efficient, sustainable, and selective solvolysis protocols is an ongoing area of research. Additionally, the integration of solvolysis reactions with other synthetic methodologies, such as catalysis and photochemistry, offers promising avenues for future exploration.
Sustainability and Green Chemistry
The application of green chemistry principles to solvolysis reactions is becoming increasingly important. This involves the use of environmentally friendly solvents, minimizing waste, and optimizing reaction conditions to reduce energy consumption. The development of solvent-free or aqueous solvolysis protocols is particularly appealing from a sustainability perspective.Solvolysis Reactions Image Gallery
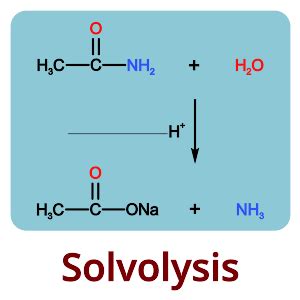
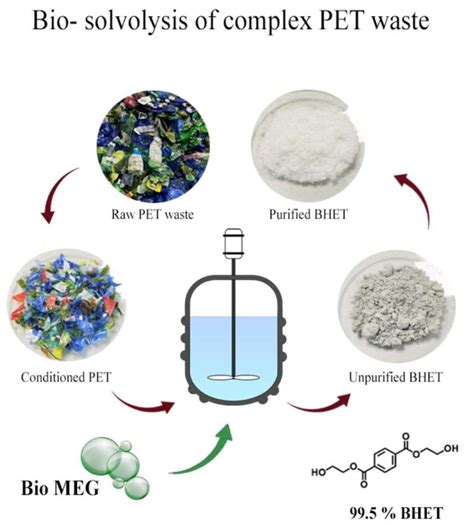
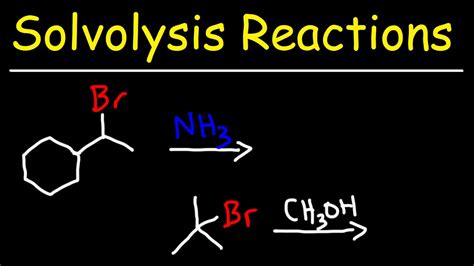

In conclusion, solvolysis reactions are a powerful tool in organic synthesis, offering a versatile method for the formation and cleavage of chemical bonds. By understanding the mechanisms, factors influencing these reactions, and their applications, chemists can harness the full potential of solvolysis to synthesize complex molecules efficiently. As research continues to advance in this field, the development of more sustainable, selective, and efficient solvolysis protocols will play a crucial role in addressing the challenges of modern organic chemistry. We invite readers to share their insights, ask questions, or discuss the applications of solvolysis reactions in their own research, fostering a collaborative environment that promotes the advancement of chemical sciences.
